Self-amplifying RNA vaccines will add to the arsenal of conventional messenger RNA jabs.Credit: Pascal Pochard-Casabianca/AFP via Getty
The approval of yet another RNA-based vaccine for COVID-19 might not seem momentous. But the endorsement last week by Japanese authorities of a jab against SARS-CoV-2 constructed using a form of RNA that can make copies of itself inside cells — the first ‘self-amplifying’ RNA (saRNA) granted full regulatory approval anywhere in the world — marks a pivotal advance.
The new vaccine platform could provide potent defence against various infectious diseases and cancers. And because it could be used at a lower dose, it might have fewer side effects than other messenger RNA (mRNA) treatments have.
Why rings of RNA could be the next blockbuster drug
When used as a booster in clinical testing, the newly authorized vaccine, ARCT-154 — developed by Arcturus Therapeutics in San Diego, California, and its partner CSL, a biotechnology firm headquartered in Melbourne, Australia — triggered higher levels of virus-fighting antibodies1 that circulated the body for longer than did a standard mRNA COVID-19 vaccine.
Researchers have been trying to make saRNA vaccines a reality for more than 20 years. “Being the first to bring an approval for this platform is pretty huge,” says Roberta Duncan, RNA-programme leader at CSL and vice-chair of the Alliance for mRNA Medicines, an advocacy organization that launched last month to advance the sector’s policy priorities.
“It’s incredibly validating to the field,” says Nathaniel Wang, chief executive and co-founder of Replicate Bioscience in San Diego, California, a company that develops saRNA vaccines. He anticipates that, with continuing advancements, saRNA technology will increasingly replace conventional mRNA in a diverse array of therapeutic contexts. “It has more versatility in its potential,” Wang says.
Amped up
That versatility emerges from its unique features.
Conventional mRNA-based COVID-19 shots consist mainly of the genetic instructions for a viral protein that are surrounded by regulatory sequences. A cell’s machinery produces the protein for as long as these instructions persist, and that protein — known as an antigen — stimulates an immune response. By contrast, saRNA jabs go a step further by integrating the genes needed for the replication and synthesis of the antigen-encoding RNA, effectively establishing a biological printing press for fabricating the vaccine inside cells (see ‘Vaccine strategies compared’).
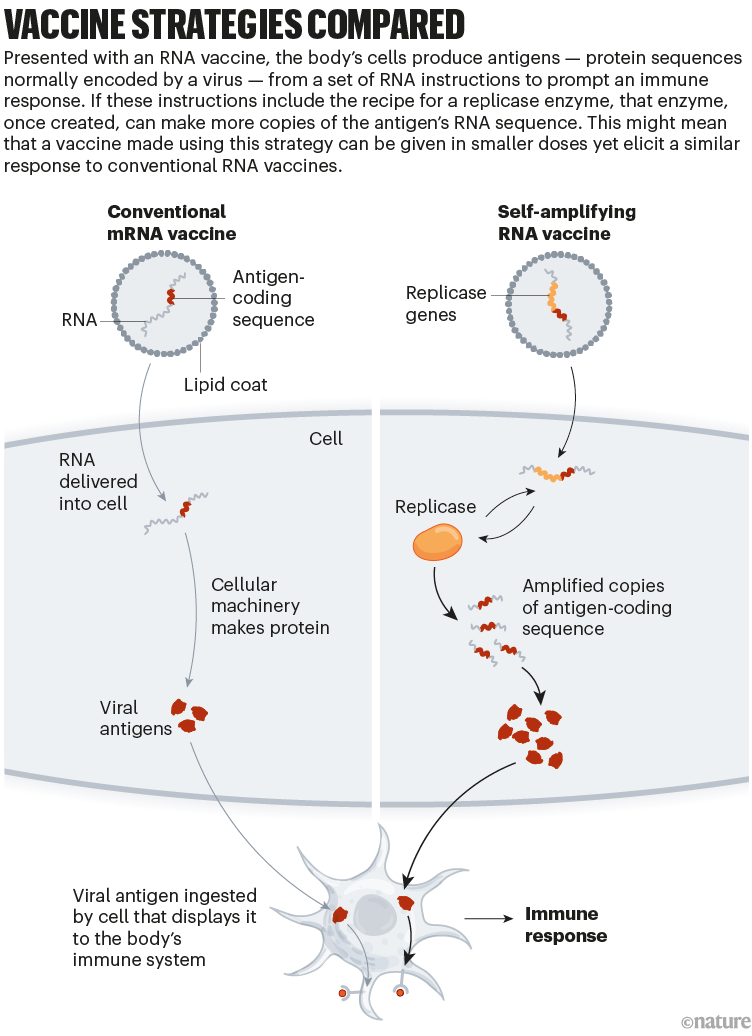
Credit: Nik Spencer/Nature, adapted from “How COVID unlocked the power of RNA vaccines”
In the case of ARCT-154, the antigen is a surface protein called spike that is expressed by SARS-CoV-2. The replication machinery is taken from a naturally occurring virus, a mosquito-borne pathogen known as Venezuelan equine encephalitis virus that causes deadly brain swelling in horses and humans. Notably, scientists at Arcturus have removed key genes from the viral sequence backbone, thus rendering the system non-infectious and safe for use in humans.
People often think that the saRNA vaccine platform is simply a variation on conventional mRNA shots, “but in practice it’s really not”, says Anna Blakney, a bioengineer who studies the technology at the University of British Columbia in Vancouver, Canada. “saRNA is a totally different beast.”
Because of its virus-like nature, saRNA interacts with the immune system in distinctive ways that could prove beneficial across a range of disease scenarios. When it comes to preventing infections, for instance, its self-amplifying capabilities could enable the use of lower vaccine doses.
ARCT-154 requires one-tenth to one-sixth as much vaccine per person as other RNA-based COVID-19 booster jabs. Reducing the amount of vaccine administered in each injection should result in lower production costs. And although the side-effect profile of ARCT-154 seems comparable to that of a conventional mRNA shot1, it is conceivable that the benefits of the platform’s smaller doses will help to mitigate the severity of aches, fevers, chills and other loathsome symptoms collectively known as reactogenicity.
These unpleasant reactions remain a considerable impediment for people to take mRNA-based vaccines. Consider the seasonal influenza vaccine. Existing jabs that use older vaccine technology cause only mild reactions. At present, several conventional mRNA-based flu jabs are progressing through clinical trials and these are showing promising signs of eliciting more protective antibodies than existing shots. Yet their side-effect profiles still leave room for improvement, notes Christian Mandl, co-founder and chief scientific officer of Tiba Biotech in Cambridge, Massachusetts. The saRNA vaccines’ “lower dose could help to solve some of the reactogenicity issues”, he says.
Big shot
The saRNA vaccine platform does have some downsides. Because of the added genetic instructions, the jabs tend to contain longer sequences — typically at least three times the length of what is used in conventional mRNA shots — which adds complexity to the manufacturing process.
Pioneers of mRNA COVID vaccines win medicine Nobel
They also engage with the immune system in intricate ways — for example, by forming replication intermediates that help to stimulate beneficial immune-signalling pathways. However, excessive stimulation can backfire, including when the vaccine prompts the immune system to block RNA replication, thereby nullifying its benefits.
It is a delicate needle to thread, says Niek Sanders, a gene-therapy researcher at Ghent University in Belgium and a scientific founder of Ziphius Vaccines, a company in Merelbeke, Belgium, that develops saRNA-based medicines. “You have to find the optimal dose of the self-amplifying RNA in combination with the right delivery system.”
The biotech industry has tried for decades to get the balance right. From 2003 to 2010, for instance, a company called AlphaVax, based in Research Triangle Park, North Carolina, conducted trials of saRNA vaccine candidates for a range of infectious diseases and cancers. AlphaVax ultimately wound down for “business reasons” after failing to secure further investment, says the firm’s co-founder Jonathan Smith, who continues to develop saRNA vaccines as the chief scientific officer of VLP Therapeutics in Gaithersburg, Maryland.
The fruits of fortitude
With approval for ARCT-154 secured in Japan, its developers are now seeking authorization in Europe; a regulatory decision is expected next year.
“This will hopefully begin to put a nail in the coffin of the idea that self-amplifying RNA is not a viable platform,” says Corey Casper, president and chief executive of the Access to Advanced Health Institute in Seattle, Washington. (Another saRNA jab for COVID-19 was approved on an emergency-use basis in India last year; however, that vaccine’s less-impressive clinical data, the provisional nature of the product’s authorization, and India’s less stringent regulatory requirements have all led industry insiders to consider ARCT-154’s approval to be the field’s true watershed moment.)
The tangled history of mRNA vaccines
More than a dozen saRNA vaccine candidates are currently in clinical trials for a range of applications — from shots for shingles and the flu to therapeutic vaccines for cancer. But researchers are already considering the platform’s broader applications.
For example, the technology might one day be used to produce therapeutic proteins inside the body, says Mark Grinstaff, a biochemist at Boston University in Massachusetts and a co-founder of Keylicon Biosciences in Brookline, Massachusetts.
Manufacturing plants currently use bioreactors to produce such proteins, which are then injected into people who need the treatment. Over the past few months, two independent groups — one involving Smith’s team at VLP Therapeutics2, the other involving Grinstaff and his colleagues at Boston University3 — have posted preprints that describe how altering the chemical backbone of saRNA can diminish the technology’s immune-triggering effects in a positive way. Similar chemical tweaks are commonly used in conventional mRNA vaccines, but not in ARCT-154 or most other saRNA products.
“People are working pretty hard” to expand the platform’s scope, says Smith. “There are some inherent advantages of saRNA — if we’re smart enough to take advantage of them.”
Source link